Research Projects

Biocompatibility Assessment of Nano-Bio Sensors
R&D Team: Dr. Steve Roberts, 1 Dr. David Barber, Dr. Scott Wasdo, Dr. Kevin Powers2, Dr. Maria Palazuelos Jorganes3, Dr. Scott Brown2, Dr. Brij Moudgil2,3,4.
1Center for Environmental and Human Toxicology, 2Particle Engineering Research Center, 3Center for NanoBio Sensors, 4Department of Materials Science and Engineering.
Opportunity and Impact: With the rapid advancement in the field of nanotechnology, there has been rising concern regarding the potential  risks associated with the widespread use of engineered nanomaterials. Because of the increasing number of nanomaterials and the wide range of applications, research associated with the potential risk of nanoparticles to biological organisms has drawn interest from academia, industry, and governmental regulatory agencies worldwide. Conducting reproducible and reliable biocompatibility studies with nanostructures is complicated by the uncertain behavior of particulate matter in biological settings and the difficulty in making in situ measurements of properties such as size, shape and surface chemistry. Because of this complexity, risk assessment of nanomaterials requires the close collaboration of experts in different fields such as toxicology, materials science, chemistry, medicine and molecular biology.
risks associated with the widespread use of engineered nanomaterials. Because of the increasing number of nanomaterials and the wide range of applications, research associated with the potential risk of nanoparticles to biological organisms has drawn interest from academia, industry, and governmental regulatory agencies worldwide. Conducting reproducible and reliable biocompatibility studies with nanostructures is complicated by the uncertain behavior of particulate matter in biological settings and the difficulty in making in situ measurements of properties such as size, shape and surface chemistry. Because of this complexity, risk assessment of nanomaterials requires the close collaboration of experts in different fields such as toxicology, materials science, chemistry, medicine and molecular biology.
Implicit in any in-vitro or in-vivo use of nano-bio sensors is the requirement that they be biocompatible with the organism or the biological tissues 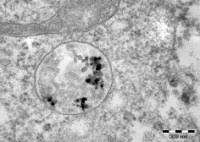 and fluids being tested. For in-vivo systems they must be non-toxic and the materials must not elicit a biological response that interferes with the test. Sensors designed for non-invasive or in-vitro use must be compatible with the biological fluids under investigation and any reagents, particles or components used for measurement. Nanoparticle mediated sensors such as bio-imaging aids, drug delivery aids or colorimetric in-vitro sensors may have additional issues such as increased dissolution rates, aggregation and surface adsorption of biological molecules which may decrease their effectiveness. The biocompatibility assessment team is organized to provide an early evaluation of proposed sensors in order to identify and address nano-bio interactions that may impact the development and commercialization of Center technologies. Expertise is also available during the testing of more mature prototypes to ensure safety and efficacy of the sensor technology.
and fluids being tested. For in-vivo systems they must be non-toxic and the materials must not elicit a biological response that interferes with the test. Sensors designed for non-invasive or in-vitro use must be compatible with the biological fluids under investigation and any reagents, particles or components used for measurement. Nanoparticle mediated sensors such as bio-imaging aids, drug delivery aids or colorimetric in-vitro sensors may have additional issues such as increased dissolution rates, aggregation and surface adsorption of biological molecules which may decrease their effectiveness. The biocompatibility assessment team is organized to provide an early evaluation of proposed sensors in order to identify and address nano-bio interactions that may impact the development and commercialization of Center technologies. Expertise is also available during the testing of more mature prototypes to ensure safety and efficacy of the sensor technology.
Project Description: A multidisciplinary group of toxicologists, materials engineers and physical scientists will provide expertise and assistance in the selection and testing of nanomaterials and nanostructures that are used in nano-bio sensors developed through the CNBS. Primary focus will be on the characterization of nanoparticles and other nanoscale materials in biological environments and the evaluation of the potential toxicity of such materials. This group of scientists and engineers will collaborate with other resources such as the Florida nanotoxicology program (Florida nTox www.nanotoxicology.ufl.edu ) to help develop safe and effective nano-bio sensors.