Research Projects

Traumatic Brain Injury Nanosensor
R&D Team: Dr. Kevin Wang1, Dr. Weihong Tan2, Dr. Firas Kobeissy1, Dr. Stephen Larner3.
1Department of Psychiatry, 2Department of Chemistry, 3Banyan Biomarkers, Inc.
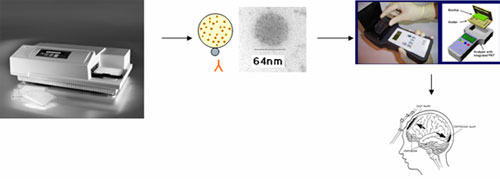
Opportunity and Impact: The development of a novel nano-biosensor-based handheld point-of-care (POC) analyzer for the ultra-sensitive detection of brain injury biomarkers in human biofluids as a result of physical trauma, blast injury or act of biological or chemical terrorism. Currently, no rapid POC device exists that is capable of detecting acute brain injury biomarkers in human biofluid such as blood. About 1.5 million patients each year (USA) suffer from a traumatic brain injury (TBI) and another 500,000 suffer a stroke, consequently there is a tremendous need and opportunity for a device that will give doctors and emergency personnel a tool that can quickly assess the extent of injury. In addition, there is appreciable amount of interest from the Department of Defense (DoD) since TBIs now make up about 2/3 of the war casualties, many of which resulted from blast injuries. Through DoD support since 2002, members of this team have already discovered several novel biomarkers for TBI and have developed antibody tools for their detection with sandwich ELISAs (one detection antibody and one capture antibody). Also, we have access to a prototype biochip that can detect a biomarker in biofluid samples using a sandwich antibody system. However, the major technology barrier has been its suboptimal detection limit (10-50 ng/mL).
Here, we submit that this technology barrier can be overcome by using dye-doped nanosphere-based biosensor with greatly enhanced fluorescent signal (over 100-fold improvement). The current funding would allow for biomarker antibody-nanosphere coupling technology to rapidly develop and be adapted to the biochip-handheld device platform similar to those available to diabetics to monitor insulin levels from blood samples and would find an immediate market within the emergency medical community, Homeland Security and DoD. As more biomarkers are discovered the device(s) can be expanded to cover all forms of pathology. We expect that once these proof-of-principle works are done, we will be in a highly competitive position to attract federal funding for small business (SBIR, STTR), venture capital or industrial partnership for further product development and commercialization.
Project Description: To work out the bioengineering difficulties of coupling molecular biomarkers with nanospheres and to develop a nano-biosensor POC device will require engineering capabilities not yet funded by external sources. Initial proof-of-concept studies will be done using fluorescence dye-doped nanosphere probes to enhance detection limit in a traditional 96-well ELISA format (Phase I), followed by the adaptation of nanosphere-antibody conjugate technology onto a prototype nano-biosensor enabled biochip/handheld POC analyzer for field testing using biofluids (cerebrospinal fluid and serum/ plasma) from animal model of acute brain injury (Phase II). We project that the final prototype product will be Nanosensor-based Biochip with Handheld POC Analyzer.